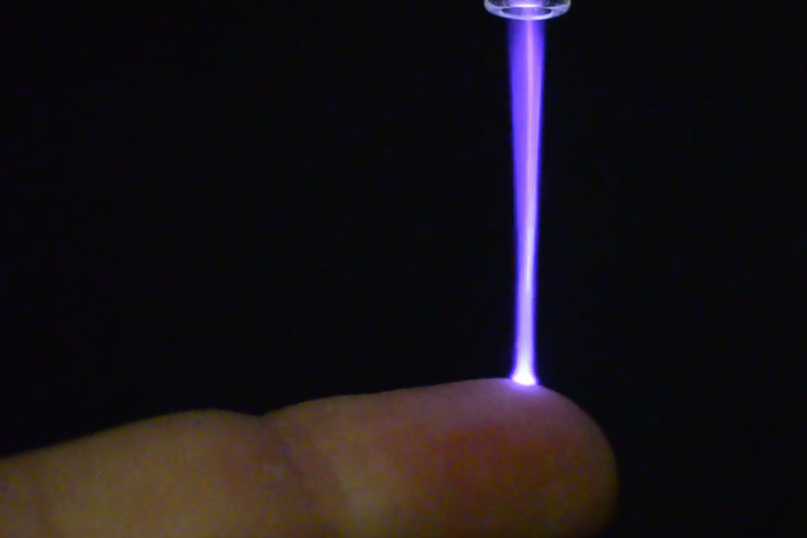
In the article “US Medical Innovations Secures FDA Clearance for Canady Helios Cold PlasmaTM Ablation System,” Yahoo! Finance shared US Medical Innovations’ announcement that they recently secured an FDA 510k Clearance for the Canady Helios Cold PlasmaTM Ablation System, for which Professor of Mechanical and Aerospace Engineering Michael Keidar contributed to developing.
Here is an excerpt from the article: “The Canady Helios Cold PlasmaTM System introduces a novel approach to soft tissue ablation using plasma. The system creates a plasma jet consisting of a pre-programmed, pulsed, non-contact, non-thermal (24 C to 30 C), three-dimensional, Plasma Treated Electromagnetic FieldTM (PTEF). This plasma jet is applied for 5 to 7 minutes intra-operatively to the microscopic soft tissue surgical margin following the surgical removal of a solid tumor.”
Read the full article on Yahoo! Finance.